ClinCare Safety Reporting Web Portal
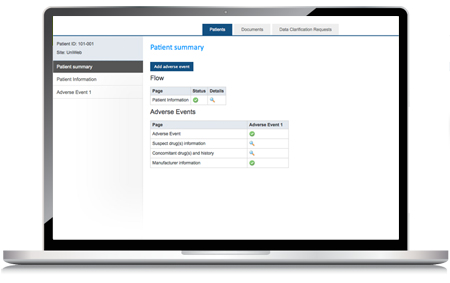
The ClinCare Safety Reporting web portal is a web-based solution for the management of adverse event and adverse reaction reporting. This tool is designed for use by manufacturers and healthcare providers as an e-reporting solution for the generation of adverse reaction reports for drugs, vaccines, devices, and combination products.
The web portal offers management tools to create, verify, validate, submit, and maintain adverse reaction reports. It creates submission ready reports that can be configured for direct reporting to both the EMA (EudraVigilance) and the FDA (FAERS). Additional reporting formats can be configured for regional and local agency requirements.
Features
- Secure web platform
- ICH E2B compliance
- Direct reporting via EudraVigilance and FDA AERS
- Extensive data validation and cross-field validation checks
- Integrated MedDRA coding
- Audit trail
- Intuitive user interface
- Customizable
Supported report formats
- CIOMS I
- FDA MedWatch
- E2B compliant XMLs
- etc..
ClinCare Safety Reporting Mobile
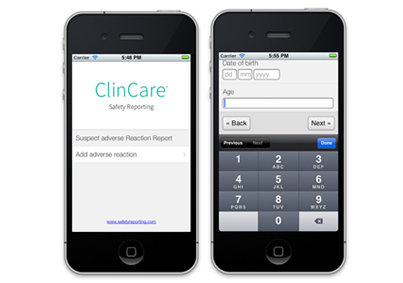
ClinCare Safety Reporting Mobile is a mobile adverse reaction reporting application. The secure mobile application guides the user through a set of intuitive and easy-to-use forms in order to collect adverse reaction information. The adverse reaction report will then be submitted to the regulatory authorities (i.e., EMA and FDA) via their mobile phone or tablet.
Safety in the palm of your hand.
Submit a report by clicking the “Add adverse reaction” button. From here, the clinician or manufacturer can inform the FDA, the EMA or both about any suspect adverse reaction from the screen of their mobile phone or tablet.
Features
- Secure mobile platform
- ICH E2B compliance
- Direct reporting via EudraVigilance and FDA AERS
- Intuitive user interface